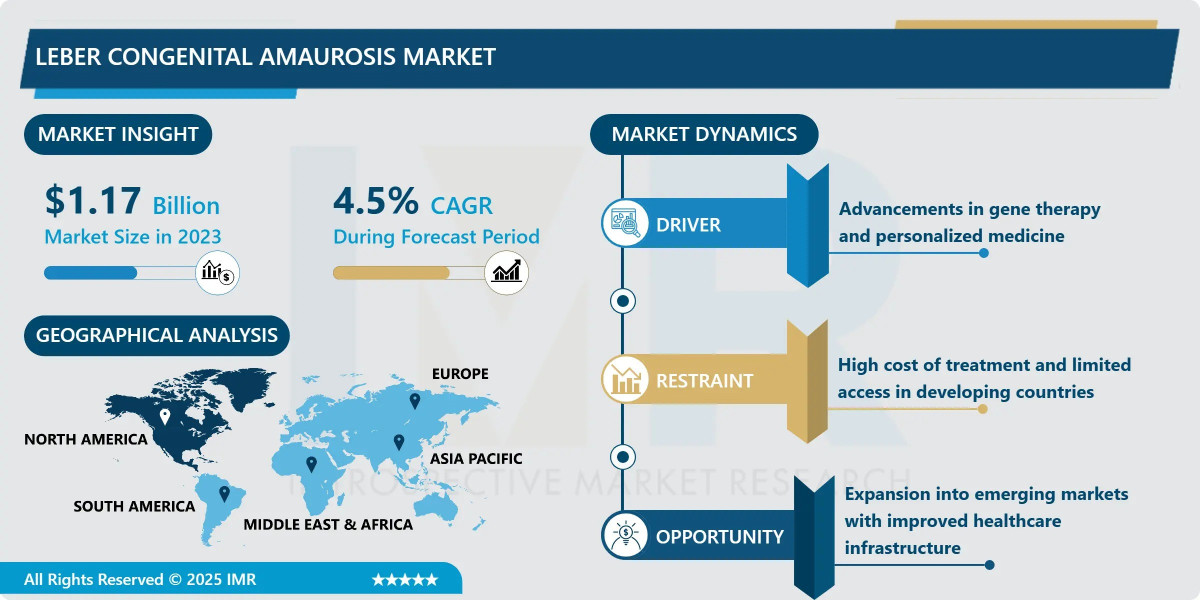
According to a new report published by Introspective Market Research, Leber Congenital Amaurosis Market by Treatment Type, Target Gene, and End User, The Global Leber Congenital Amaurosis Market Size Was Valued at USD 1.17 Billion in 2023 and is Projected to Reach USD 1.74 Billion by 2032, Growing at a CAGR of 4.5%.
Introduction / Market Overview: Leber Congenital Amaurosis (LCA) is a rare group of inherited retinal diseases characterized by severe visual impairment or blindness at birth or within the first few months of life. It is primarily caused by mutations in genes essential for the normal development and function of photoreceptor cells and the retinal pigment epithelium. For decades, the market was limited to supportive care and low-vision aids. However, the emergence of targeted gene therapies has transformed the landscape, offering the possibility of actual vision restoration for the first time in history.
The LCA market is a pioneer in the field of precision medicine, particularly in gene replacement therapy. By delivering functional copies of defective genes directly to the retina, these treatments address the root cause of the disorder. With advancements in genetic testing and increased awareness of inherited retinal dystrophies, more patients are being accurately diagnosed at an early age, creating a stable and growing demand for specialized ophthalmic interventions.
Market Segmentation: The Leber Congenital Amaurosis Market is segmented into Treatment Type, Target Gene, and End User. By Treatment Type, the market is categorized into (Gene Therapy, Pharmaceutical Drugs, Retinal Prosthesis, Assistive Devices). By Target Gene, the market is categorized into (RPE65, GUCY2D, CEP290, AIPL1, Others). By End User, the market is categorized into (Hospitals, Specialized Eye Clinics, Ophthalmology Research Centers, Others).
Growth Driver: The primary growth driver for the Leber Congenital Amaurosis market is the breakthrough success and adoption of gene therapy products. The approval of voretigene neparvovec (Luxturna) established a commercial and clinical blueprint for treating monogenic retinal diseases, proving that a single-dose subretinal injection can provide long-lasting benefits. This success has spurred heavy investment into the R&D pipeline, with numerous candidates targeting different LCA-causing genes now in late-stage clinical trials. Additionally, the increasing availability of affordable genetic screening allows for earlier diagnosis, which is crucial for the timely administration of these specialized therapies.
Market Opportunity: A significant market opportunity lies in the expansion of CRISPR/Cas9 and RNA-based therapies for LCA mutations that are not easily addressed by standard gene replacement. For example, large genes like CEP290 (LCA10) are too big for traditional viral vectors, making them ideal targets for gene editing or antisense oligonucleotides. Furthermore, emerging markets in the Asia-Pacific and Latin American regions represent untapped potential. As healthcare infrastructure improves and precision medicine becomes more accessible in these regions, the patient pool eligible for high-value gene therapies is expected to grow substantially.
Detailed Segmentation:
Title: Leber Congenital Amaurosis Market, Segmentation The Leber Congenital Amaurosis Market is segmented on the basis of Treatment Type, Target Gene, and End User.
Treatment Type The Treatment Type segment is further classified into Gene Therapy, Pharmaceutical Drugs, Retinal Prosthesis, and Assistive Devices. Among these, the Gene Therapy sub-segment accounted for the highest market share in 2023. Gene therapy is the most revolutionary segment of this market, offering a curative approach rather than just symptom management. Its dominance is driven by high treatment costs and the high clinical efficacy of approved products like Luxturna. Since gene therapy directly targets the biallelic RPE65 mutation to restore the visual cycle, it is increasingly considered the primary treatment option for eligible patients.
Target Gene The Target Gene segment is further classified into RPE65, GUCY2D, CEP290, and AIPL1. Among these, the RPE65 sub-segment accounted for the highest market share in 2023. Mutations in the RPE65 gene were the first to be successfully targeted with an FDA-approved gene therapy, making this segment the most mature in terms of commercial revenue. Research into RPE65-mediated LCA has been extensive for over a decade, resulting in a well-established patient identification pathway and strong clinical data supporting the long-term safety and efficacy of RPE65-targeted interventions.
Some of The Leading/Active Market Players Are-
· Spark Therapeutics (Roche) (US)
· Novartis AG (Switzerland)
· Biogen Inc. (US)
· GenSight Biologics (France)
· AbbVie Inc. (US)
· Regeneron Pharmaceuticals (US)
· Pfizer Inc. (US)
· Astellas Pharma (Japan)
· Santen Pharmaceutical (Japan)
· MeiraGTx (UK/US)
· Opus Genetics (US)
· Atsena Therapeutics (US)
· Johnson & Johnson Innovative Medicine (US)
· ProQR Therapeutics (Netherlands)
· Ocugen, Inc. (US) and other active players.
Key Industry Developments
News 1: In November 2024, Atsena Therapeutics entered into an exclusive licensing agreement with Nippon Shinyaku for the development and commercialization of ATSN-101. This investigational gene therapy is designed for the treatment of Leber Congenital Amaurosis type 1 (LCA1). The partnership aims to accelerate the Phase 3 clinical trials and eventual market entry in the US and EU. ATSN-101 targets the GUCY2D gene and has shown promising results in early trials by improving retinal sensitivity and the ability of patients to navigate low-light environments.
News 2: In October 2025, Opus Genetics reported significant vision improvements in its Phase 1/2 gene therapy clinical trial for LCA5. The company announced that patients who were almost totally blind since birth were able to identify objects for the first time. Following these positive results, the company plans to enroll pediatric patients in early 2025. LCA5 is one of the most severe forms of the disease, and these findings provide hope for a previously untreatable patient population, further validating the potential of gene augmentation therapies.
Key Findings of the Study
· Gene therapy remains the dominant treatment type due to its curative potential and the high value of approved therapies.
· The RPE65 target gene segment holds the largest share, but candidates for CEP290 and GUCY2D are rapidly advancing.
· Hospitals and specialized eye clinics are the primary end users, managing complex subretinal injection procedures.
· Increasing government support for rare disease research is a major driver for market expansion and pipeline growth.