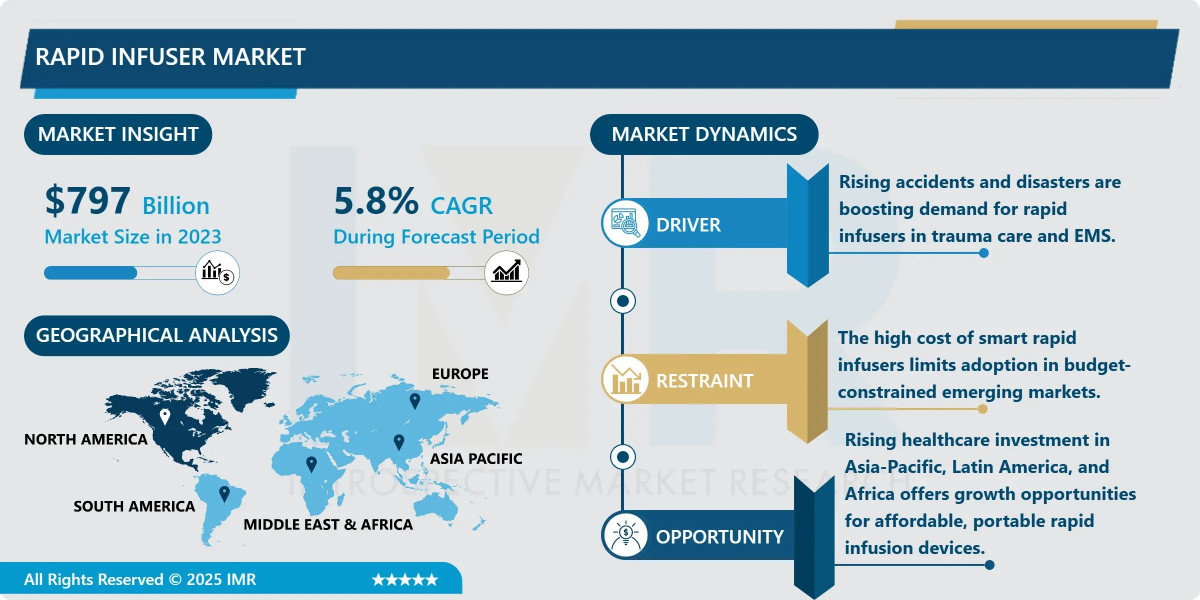
According to a new report published by Introspective Market Research, Rapid Infuser Market by Product Type, Application, End-User, and Region, The Global Rapid Infuser Market Size Was Valued at USD 797 Million in 2023 and is Projected to Reach USD 1,113 Million by 2032, Growing at a CAGR of 5.8%.
Market Overview:
The global rapid infuser market encompasses critical medical devices designed for the rapid, large-volume infusion of warmed intravenous (IV) fluids or blood products. These systems are engineered to deliver life-saving fluids at high flow rates—often exceeding 1000 mL per minute—while simultaneously warming the fluid to body temperature to prevent hypothermia, a key risk in major trauma and surgery. Compared to traditional manual infusion methods or standard IV pumps, rapid infusers offer unparalleled advantages in speed, temperature management, and safety, directly addressing the "golden hour" in emergency resuscitation where time is critical.
These devices are indispensable in high-acuity clinical settings, with their main applications centered around emergency and trauma care, major surgical procedures (especially cardiac, transplant, and orthopedic surgeries), and critical care units. They are essential for managing conditions like severe hemorrhage, septic shock, and massive transfusion protocols. The ability to prevent complications such as coagulopathy and cardiac arrhythmias, which are associated with the infusion of cold fluids, underscores their clinical value. The market's growth is fundamentally driven by the rising global incidence of trauma cases, advancements in complex surgical procedures requiring precise fluid management, and the increasing implementation of standardized resuscitation protocols in hospitals worldwide.
Growth Driver:
The primary growth driver for the rapid infuser market is the increasing global burden of trauma and emergency cases, coupled with the stringent adoption of advanced resuscitation guidelines. Rising incidences of road accidents, injuries from conflicts, and other traumatic events necessitate immediate, high-volume fluid replacement to prevent hypovolemic shock. Modern trauma and Advanced Cardiac Life Support (ACLS) protocols increasingly mandate the use of rapid infusion systems as a standard of care to ensure precise, fast, and warmed fluid delivery. This clinical imperative, supported by evidence demonstrating improved patient survival and reduced complication rates, is compelling healthcare facilities globally to invest in and upgrade their emergency response infrastructure with these life-saving devices.
Market Opportunity:
A significant market opportunity lies in the expansion into non-traditional and pre-hospital care settings, such as military field hospitals, air ambulances (medevac), and mobile ICUs. The development of next-generation portable, battery-operated, and ruggedized rapid infusers is creating new avenues for market growth. These compact systems are designed to function reliably in challenging environments outside the controlled hospital setting, enabling advanced resuscitation to begin at the point of injury or during transport. This trend aligns with the broader shift towards decentralized and faster emergency care, opening substantial demand from defense organizations, emergency medical services (EMS), and disaster response units seeking to enhance their frontline medical capabilities.
Rapid Infuser Market, Segmentation
The Rapid Infuser Market is segmented on the basis of Product Type, Application, and End-User.
Application
The Application segment is further classified into Emergency & Trauma Care, Operating Rooms, Intensive Care Units, and Others. Among these, the Emergency & Trauma Care sub-segment accounted for the highest market share in 2023. This dominance is rooted in the critical, time-sensitive nature of trauma resuscitation, where rapid blood and fluid administration is paramount to survival. Rapid infusers are often the first-line devices in trauma bays and emergency departments to manage hemorrhagic shock, directly impacting mortality rates. The high volume of trauma cases worldwide and the protocol-driven adoption of these devices in Level I trauma centers solidify this segment's leading position in market revenue.
End-User
The End-User segment is further classified into Hospitals, Ambulatory Surgical Centers (ASCs), and Specialty Clinics. Among these, the Hospitals sub-segment accounted for the highest market share in 2023. Hospitals, particularly large tertiary care centers and academic teaching hospitals with dedicated trauma, cardiac, and transplant surgery departments, are the primary adopters. They possess the high patient volume, complex case mix, and financial resources necessary for investing in advanced capital equipment like rapid infusers. The concentration of emergency rooms, ICUs, and major operating suites within a single facility drives consolidated, high-value procurement and utilization, ensuring hospitals remain the central revenue-generating endpoint for this market.
Some of The Leading/Active Market Players Are-
- Stryker Corporation (USA)
• 3M Company (USA)
• Smiths Medical (USA)
• Belmont Medical Technologies (USA)
• Becton, Dickinson and Company (USA)
• GE Healthcare (USA)
• Teleflex Incorporated (USA)
• Zimmer Biomet (USA)
• Fresenius Kabi AG (Germany)
• B. Braun Melsungen AG (Germany)
• Baxter International Inc. (USA)
• Micrel Medical Devices (Greece)
• Ace Medical Devices (USA)
• Vyaire Medical, Inc. (USA)
• InfuSystem Holdings, Inc. (USA)
• and other active players.
Key Industry Developments
News 1:
In March 2024, Stryker launched its next-generation Ranger® High-Flow Fluid Warmer with enhanced connectivity for integration into hospital IoT networks. This allows for remote monitoring of infusion parameters and automated data logging into electronic health records, streamlining clinical workflows and improving compliance with transfusion protocols in high-stakes environments.
News 2:
In November 2023, Belmont Medical Technologies received FDA clearance for a new portable rapid infuser system specifically designed for military and EMS use. The device features extended battery life, ruggedized construction, and intuitive operation for use in austere environments, aiming to bring advanced resuscitation capabilities closer to the point of injury.
Key Findings of the Study
- Emergency & Trauma Care is the dominant application segment due to the critical need for rapid resuscitation.
• North America leads the regional market, supported by advanced healthcare infrastructure and high adoption of trauma protocols.
• The rising global incidence of trauma and complex surgeries is the key growth driver.
• A major trend is the development of portable, ruggedized systems for pre-hospital and military applications, expanding the market's reach.